Preparation of alginate hydrogel microspheres with Microdroplets /Microsphere Generator (by ion-exchange cross-linking gelation)
In this application note, alginate hydrogel microspheres with high monodispersity are prepared with Microdroplet/Microsphere Generator. Drop-Surf Droplet Generation Oil is used as a continuous phase and 2 aqueous phases (chelated Ca-EDTA and sodium alginate, chelated Zn-EDDA and sodium alginate) are used as dispersed phases, and the microdroplets are cross-linking gelation by ion-exchange to obtain highly monodisperse alginate hydrogel microspheres (CV<5%).
Hydrogels are an important class of soft materials with far ranging applications in drug research, drug delivery, tissue engineering, food and pharmaceutical sciences. From a vast library of hydrogel forming (bio)polymers, alginate, as a natural polysaccharide, has attracted great attention in the biomedical field because of its low toxicity, mild ionic cross-linking conditions, good biocompatibility and biodegradability[1]. Currently, it is reported that micrometer-sized alginate particles act as bioscaffolds for 3D cell culture units to simulate the matrix environment of cell growth, which are widely used for the encapsulation, culture and monitoring of living cells in pharmaceutical research and tissue engineering[2]. Uyen N.T.T et al. found that alginate microgels particles can not only control the release rate of drugs, but also deliver drugs to specific therapeutic targets, and alginate can be directly degraded and excreted from the body through metabolism after drug release [3]. Typically, alginate microgels particles are prepared by ionic cross-linking of alginate solution with divalent cations such as Ca2+, Ba2+, Cu2+, Cd2+ and Sr2+ after the formation of droplets. For example, an aqueous sodium alginate is dropped into excess calcium chloride aqueous solution, and alginate microgels are prepared by stirring or ultrasound [4]. However, the microgels produced by this method have some shortcomings such as poor monodispersity of particle size and irregular shape.
Alginate microgels particles with precise control over size, shape and morphology can be produced by droplet-based microfluidics. Generally, an aqueous sodium alginate solution is emulsified and dispersed in oil phase in microfluidic device, and cross-linked gelation with Ca2+. This fast reaction of cross-linking occurs immediately and typically results in clogging and nonuniform drop formation. To overcome these problems, the generation of droplets and cross-linking gelation should be separated. Zhang et al. reported that CaCO3 nanoparticles are dispersed in the alginate solution, and can be dissolved under acidic conditions after drop formation[5]. However, the dissolution of solid CaCO3particles causes a heterogeneous distribution of Ca2+ inside the droplets and diminishes the homogeneity of the resulting particles. In addition, the agglomeration of nanoparticles will also lead to the blockage of the small microfluidic channels. Therefore, Utech et al. introduced chelated Ca-EDTA as a cross-linking precursor that allows Ca2+ to homogenously distribute alginate aqueous solution before droplets formation. Subsequently, Ca2+ may be released by pH reduction by introduction of an acid, resulting in essentially instantaneous gelation [6]. However, the pH must be reduced below ~ pH 5 to release Ca2+ which may be prohibitive for cell or protein encapsulation.
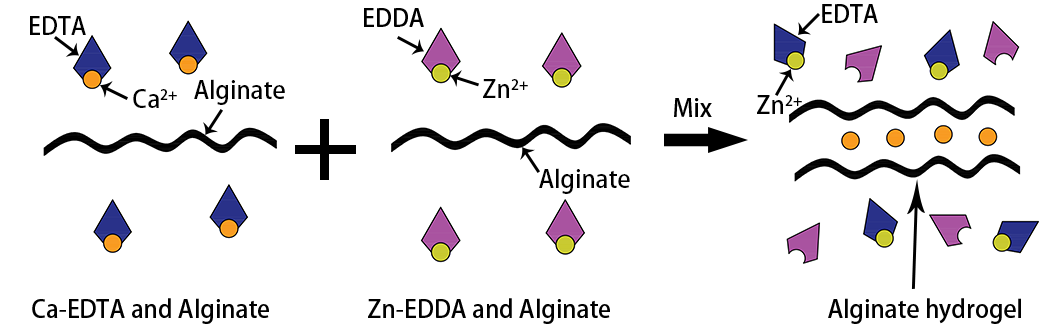
To address the above problem, FluidicLab provides an effective strategy to control the release of Ca2+ into alginate aqueous solution to form hydrogel particles by ion-exchanging. The schematic diagram is shown in the follow picture: the dispersed phase 1 (containing Ca-EDTA chelate and sodium alginate), the dispersed phase 2 (containing Zn-EDDA chelate and sodium alginate) and the continuous phase (Drop-Surf Droplet Generation Oil) are pushed into a microfluidic chip by FluidicLab Microdroplet Generator, and droplets are generated after the dispersed phases mixed together and sheared by the continuous phase. Once two dispersed phases are mixed, the Zn2+ binds with EDTA due to its affinity with different ligands, resulting in release of Ca2+ which subsequently cross-links the alginate.
Materials and equipment:
| Reagents | Droplet Generation Oil (Drop-Surf, 2% surfactant by weight) |
| Demulsifier (Drop-Surf) | |
| Alginate (FluidicLab) | |
| Anhydrous calcium chloride (Macklin, C805228-100 g) | |
| EDTA solution (Macklin, 0.5 M, pH 7.4, E885215-1L) | |
| Ethylenediamine-N, N ′-diacetic acid (EDDA, Macklin, E838453-5 G) | |
| Anhydrous zinc acetate (Macklin, E820824-25 G) | |
| Sodium hydroxide (Macklin, S817971-500 G) | |
| PBS buffer powder (Macklin, P854529-10EA) | |
| Equipment | Microdroplet/Microsphere Generator (Fluidiclab, 3 pressure channels) |
| An air compressor with an air treatment device | |
| An optical microscope for particle size measurement | |
| A high-speed centrifuge (Hunan Xiangyi, H1850) | |
| A personal computer (PC, WINDOWS 7, or 10, or 11) | |
| A pH Meter (Mettler TOLEDO, Standard Edition) | |
| A standard PDMS chip holder (FluidicLab) | |
| PDMS-SCE-100/50/30 μm chips (FluidicLab) | |
| Centrifuget ubes (1.5 mL /2 mL) | |
| Centrifuge tubes (Falcon, 15 mL REF 352097, 50 mL REF 352098) | |
| Polyurethane tubing (PU, 4 mm outer diameter, 6 mm outer diameter) | |
| Poly (enther-ether-ketone) tubing (PEEK, 1.6 mm out diameter) and connectors (1/4-28 UNF) | |
| Syringe tip filters (25 mm diameter, 0.22 μm, PTFE membrane) | |
| Syringe (10 mL) |
Experimental Procedure:
1.Solution preparation:
(1) 2 M CaCl2 aqueous solution:
2.2197 g of CaCl2 (MCaCl2=110.984 g/mol) is dissolved into pure water. Fix the volume to 10 mL.
(2) 1.2% (wt%) sodium alginate solution:
0.12 g of sodium alginate powder and 9.98 g of pure are mixed homogeneously and heated to 60℃ to be dissolved completely.
(3) Preparation of 2 M NaOH solution:
1.6 g of NaOH (MNaOH= 39.997 g/moL) is dissolved into pure water. Fix the volume to 20 mL.
(4) 80 mM Ca-EDTA solution (pH=6.7):
400 μL of 2M CaCl2, 1.6 mL of 0.5 M EDTA and 5 mL of pure water are mixed homogeneously. Add 2M NaOH into the solution to adjust pH to 6.7, finally fix the volume to 10 mL.
[Note] The volume of NaOH added in the following table is for reference only. You should determine the volume of NaOH solution based on your own measurements.
| No. | 2 M NaOH volume (μL) | pH |
| 1 | 0 | 3.92 |
| 2 | 200 | 4.38 |
| 3 | 150 | 5.97 |
| 4 | 5 | 6.21 |
| 5 | 3 | 6.38 |
| 6 | 2 | 6.51 |
| 7 | 1 | 6.58 |
| 8 | 0.5 | 6.63 |
| 9 | 0.5 | 6.67 |
| Total volume | 362 | / |
(5) 80 mM Zn-EDDA solution (pH=6.7):
0.1465 g of Zn(CH3CHO2)2 (MZN(CH3CHO2)2=183.48 g/mol), 0.1416 g of ethylenediamine-N, N’- diacetic acid (EDDA, MEDDA=176.17 g/moL) and 5 mL of pure water are mixed homogeneously. Add 2M NaOH into the solution to adjust the pH to 6.7, finally fix the volume to 10 mL.
[Note] The volume of NaOH added in the following table is for reference only. You should determine the volume of NaOH solution based on your own measurements.
| No. | 2 M NaOH volume (μL) | pH |
| 1 | 0 | 3.96 |
| 2 | 500 | 5.01 |
| 3 | 200 | 5.71 |
| 4 | 40 | 6.06 |
| 5 | 20 | 6.44 |
| 6 | 3 | 6.53 |
| 7 | 2 | 6.62 |
| 8 | 1 | 6.65 |
| 9 | 0.5 | 6.68 |
| Total volume | 766.5 | / |
(6) Preparation of dispersed phase 1 (0.6% sodium alginate, 40 mM Ca-EDTA):
A mixture of an equal volume of 80 mM Ca-EDTA solution and 1.2% sodium alginate solution is vortexed homogeneously to obtain dispersed phase 1. Filter the solution with a 0.22 μm syringe tip filter.
(7) Preparation of dispersed phase 2 (0.6% sodium alginate, 40 mM Zn-EDDA):
A mixture of an equal volume of 80 mM Zn-EDDA solution and 1.2% sodium alginate solution is vortex homogeneously to obtain dispersed phase 2. Filter the solution with a 0.22 μm syringe tip filter.
2.Preparation of microdroplets:
(1) Microfluidic devices set-up
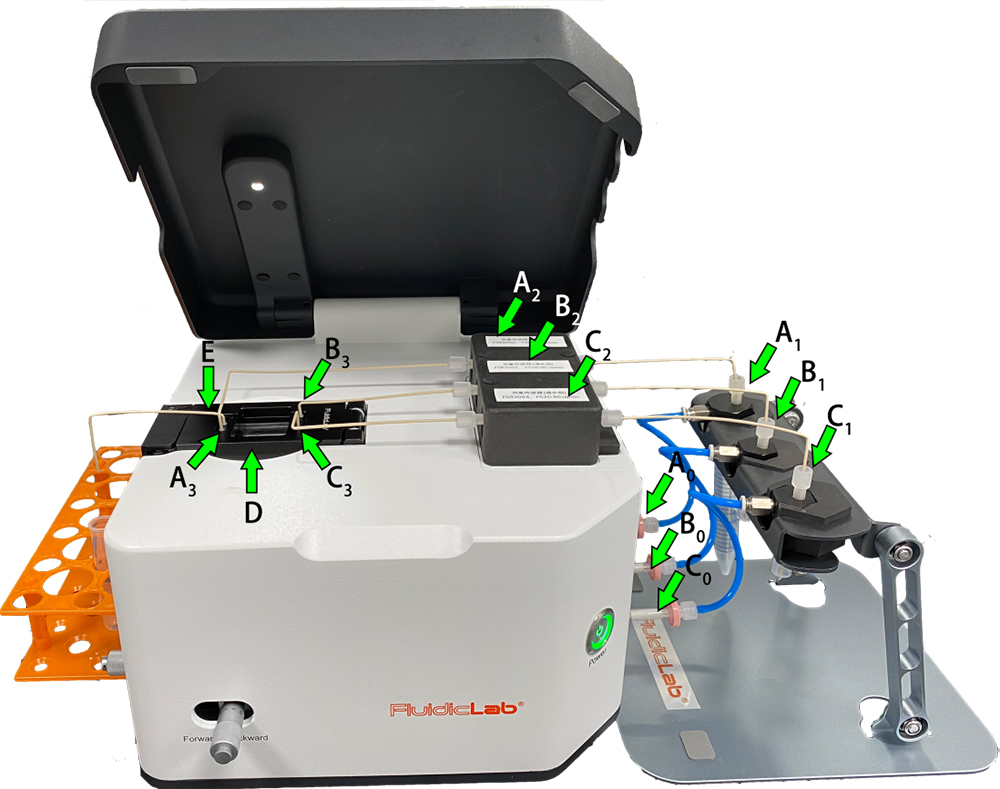
For a more detailed installation and connection of the Microdroplet/Microsphere Generator, refer to the User Guide V.1.0 Of Microdroplet/Microsphere Generator, section 2. Installation and connection of Microdroplet/Microsphere Generator. The connection is as follows (steps③-⑦ are shown in the figure below):
① Connect the devices with PU tubing in the following order:
The air compressor–the air source processing device–the Microdroplet/Microsphere Generator.
② Connect the device to the power supply and PC, respectively.
③ Connect A0 (the pressure channel 1 outlet) and A1 (the continuous phase reservoir), B0 (the pressure channel 2 outlet) and B1 (the dispersed phase 1 reservoir), C0 (the pressure channel 3 outlet) and C1 (the dispersed phase 2 reservoir) with PU tubing, respectively;
④ Connect A1 (the continuous phase reservoir) and A2 (the flow sensor channel 1 ), B1 (the dispersed phase 1 reservoir) and B2 (the flow sensor channel 2), C1 (the dispersed phase 2 reservoir) and C2 (the flow sensor channel 3) with PEEK tubing and 1/4-28 UNF, respectively;
⑤ Connect A2 (the flow sensor channel 1) and A3 (the continuous phase inlet of the PDMS chip), B2 (the flow sensor channel 2) and B3 (the dispersed phase 1 inlet of the PDMS chip), C2 (the flow sensor channel 3) and C3 (the dispersed phase 2 inlet of the PDMS chip) with PEEK tubing and 1/4-28 UNF, respectively;
⑥ D is a combination of a PDMS standard chip and a chip holder, which is sealed by 6 silicone connector seals;
⑦ The PEEK tubing is inserted into the chip & chip holder outlet (E) for emulsion output .
(2) Installition of FluidicLabSuite software:
Refer to the section 3.1 Installation of FluidicLabSuite Software in the User Guide V.1.0 Of Microdroplet/Microsphere Generator;
(3) Preparation of chitosan microdroplets:
For a visualized guide of this section, refer to Application Video: Preparation of alginate hydrogel microspheres with Microdroplet/Microsphere Generator (by ion-exchange cross-linking gelation).
① 5 mL of Droplet Generation Oil, 2 mL of dispersed phase 1 and 2 mL of dispersed phase 2 are added into the corresponding reservoirs, respectively:
Reservoir 1 (controlled by Pressure Channel 1): Droplet Generation Oil;
Reservoir 2 (controlled by Pressure Channel 2): dispersed phase 1;
Reservoir 3 (controlled by Pressure Channel 3): dispersed phase 2.
② For the use of the camera and the flow sensors, refer to the section 3.2 Equipment Addition of FluidicLabSuite Software in the User Guide V.1.0 Of Microdroplet/Microsphere Generator.
③ Turn on the air compressor and air source treatment device.
④ A Centrifuge tube is placed at the emulsion outlet to collect the pre-waste liquid.
⑤ Set the pressure of pressure channel 1, 2 and 3 by FluidicLabSuite control to exhaust the air in the PEEK tubing and the microchannels of chip.
Tips: Due to its high viscosity, we kindly suggest you set a higher pressure in the dispersed phases 1 and 2 than in the continuous phase. For example:
Pressure Channel 1 (controlling the continuous phase): 250 mbar;
Pressure Channel 2 (controlling the dispersed phase 1): 350 mbar;
Pressure Channel 3 (controlling the dispersed phase 2): 350 mbar.
⑥ After the PEEK tubing and the microchannels of chip are filled with liquid, switch the control mode from pressure control to flow rate control. The flow rates of channels 1, 2 and 3 are set as 20, 5 and 5 μL/min, respectively.
⑦ The smooth output of target flow rates can be quickly achieved through adjusting the feedback value.
⑧ After a few minutes, collect a drop of the emulsion onto a hydrophobic substrate base, and ensure the uniformity of droplet size with an optical microscope.
⑨ After that, collect the emulsion into a 1.5 mL Centrifuge tube.
⑩ Collect the emulsion for 20 minutes, and 10 minutes of cross-linking time is allowed for cross-linking gelation.
3. Aftertreatments:
For a visualized guide of this section, refer to Application Video: Preparation of alginate hydrogel microspheres with Microdroplet/Microsphere Generator (by ion-exchange cross-linking gelation).
① Remove the oil phase (at the bottom of the container) with a pipette;
② Add 2x volume of Drop-Surf Demulsifier to the microspheres. For every 100 μL microspheres, add 200 μL Drop-Surf Demulsifier;
③ Vortex the mixture for 20 seconds, then centrifuge 1000 rpm for 30 seconds. Remove the demulsifier at the bottom of the container.
④ Repeat steps ② and ③ 1~2 times, until all white microspheres on the top of the container change into transparent.
⑤ Add 3x volume of PBS buffer to the microspheres. For every 100 μL microspheres, add 300 μL PBS buffer.
⑥ Vortex the mixture for 20 seconds, then centrifuge 1000 rpm for 120 seconds. Remove PBS buffer at the upper of the container.
⑦ Repeat steps ⑤ and ⑥ 1~2 times.
⑧ Finally, the solidified alginate hydrogel microspheres are obtained and dispersed in PBS buffer.
4. Cleaning of Microdroplets/Microsphere of Generator:
The PEEK Tubing, the flow sensors and the microchannels of the chip in the instrument should be cleaned after the experiment. Otherwise, the reagents remaining in the flow channels could possibly damage the flow sensors and clog the microchannels of the chip. For details, please refer to the Instruction Card of Microdroplet/Microsphere Generator.
Results and discussion
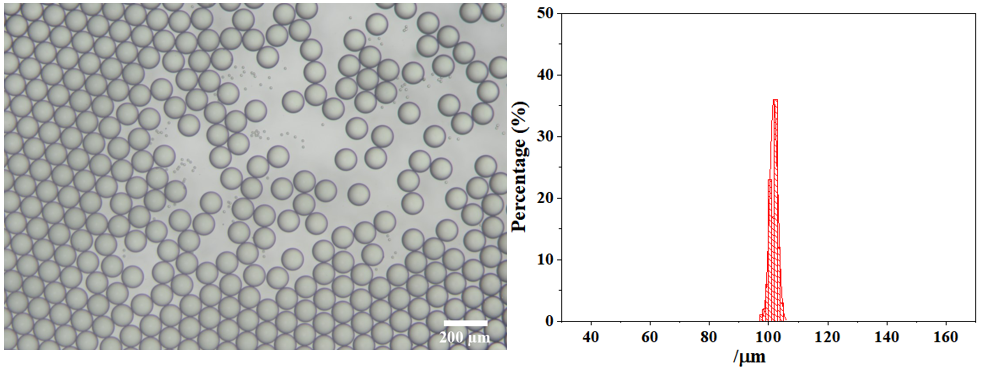
The average particle size of the microdroplets is 101.74 μm with extremely high monodispersity (coefficient of variation: CV=1.30%). The picture and particle size distribution are shown as follow:
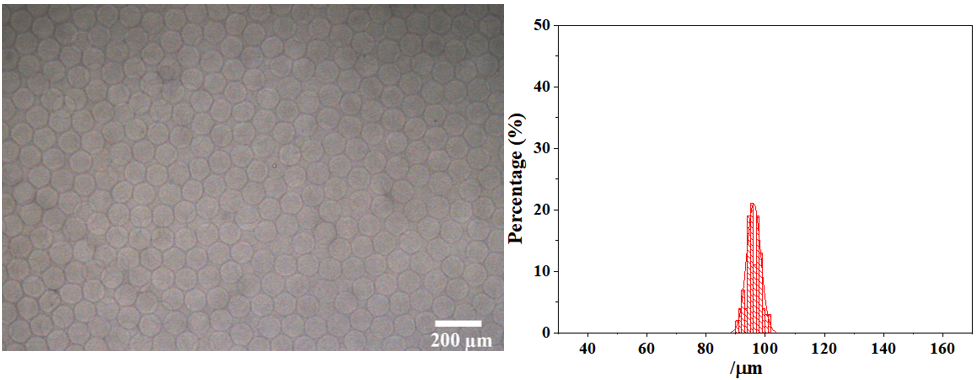
After cross-linking gelation, the average particle size of alginate hydrogel microspheres is 96.08 μm, which also has extremely high monodispersity (CV=2.52%). The picture and particle size distribution are shown as follow:
There are some different types of chips for the preparation of alginate hydrogel microspheres (1% alginate, 40 mM Ca-EDTA, 40 mM Zn-EDDA). The results are shown as follow:
| Chip | Continuous phase | Dispersed phase 1 | Dispersed phase 2 | Droplet size (μm) | CV (%) | Particle size (μm) | CV (%) | |||
| Flow rate (μL/min) | Pressure(mbar) | Flow rate (μL/min) | Pressure (mbar) | Flow rate (μL/min) | Pressure(mbar) | |||||
| PDMS-SCE-100 | 10 | 85 | 1.5 | 366 | 1.5 | 366 | 101.68 | 1.69 | 107.24 | 1.61 |
| 10 | 114 | 4.5 | 855 | 4.5 | 859 | 112.39 | 1.99 | 110.54 | 2.03 | |
| 20 | 166 | 5 | 959 | 5 | 944 | 100.60 | 1.81 | 103.16 | 1.76 | |
| 20 | 176 | 6.5 | 1110 | 6.5 | 1081 | 106.57 | 1.72 | 107.24 | 1.71 | |
| PDMS-SCE-50 | 4 | 174 | 1 | 1145 | 1 | 1157 | 53.83 | 2.66 | 58.39 | 4.10 |
| 4 | 251 | 2 | 1727 | 2 | 1658 | 60.07 | 2.49 | 65.94 | 3.37 | |
| 8 | 309 | 2 | 1700 | 2 | 1663 | 51.28 | 2.62 | 55.81 | 3.99 | |
| PDMS-SCE-30 | 5 | / | 2 | / | 2 | / | 33.72 | 2.81 | 34.42 | 3.46 |
Key points of the experiment
1.This application note is only suitable for the alginate kit provided by FluidicLab for encapsulation of cells, microbiological or macromolecular polypeptide drugs.
2.In this application note, both two dispersed phases should not contain free Ca2+. For example, a medium contain free Ca2+ is not applicable for use in the dispersed phase .
3.The viscosity of the dispersed phases is higher than that of the continuous phase, thus, the pressure of the dispersed phases is higher than that of the continuous in the same flow rate. Due to its high viscosity, we kindly suggest you set a higher pressure in the dispersed phases than in the continuous phase.
4.When there is no flow sensor on the Microdroplet/Microsphere Generator, the two dispersed phases should be equal to ensure the similar flow rates of two dispersed phases.
5.Due to its low concentration, the final obtained microspheres may not be observed when dispersed in PBS buffer. You can add a small amount of free Ca2+ into the PBS buffer, or disperse the microspheres into the water.
6.In this application note, with a higher concentration of Ca-EDTA, Zn-EDDA and sodium alginate, the final obtained outline of microspheres can be more clear and obvious.
References
[1] Ching S. H., et al. Alginate gel particles–a review of production techniques and physical properties, Crit. Rev. Food Sci., 57, 1133-1152 (2015).
[2] Andersen T., et al. 3D cell culture in alginate hydrogels, Microarrays, 4, 133-161 (2015).
[3] Uyen N.T.T., et al. Fabrication of alginate microspheres for drug delivery: a review,Int. J. Biol. Macromol., 153, 1035-1046 (2020).
[4] Rajaonarivony M., et al. Development of a New Drug Carrier Made from Alginate,J. Pharm. Sci-US, 82, 912-917 (1993).
[5] Zhang H., et al. Exploring microfluidic routes to microgels of biological polymers, Macromol. Rapid Comm.,28, 527-538 (2007).
[6] Utech S., et al. Microfluidic generation of monodisperse, structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture,Adv. Heal. Mater., 4, 1628-1633 (2015).
[7] Håti A. G., et al. Versatile, cell and chip friendly method to gel alginate in microfluidic devices, Lab Chip, 16, 3718(2016).
[8] Bassett D. C., et al. Competitive ligand exchange of crosslinking ions for ionotropic hydrogel formation, J.Mater. Chem. B, 4, 6175(2016).
GET IN TOUCH
CONTACT
Dedicated to providing professional standard microfluidic integrated solutions
-
315, Building 1, No. 8 Memorial Road, Yangpu District, Shanghai, China
-
+86 021-65103566
